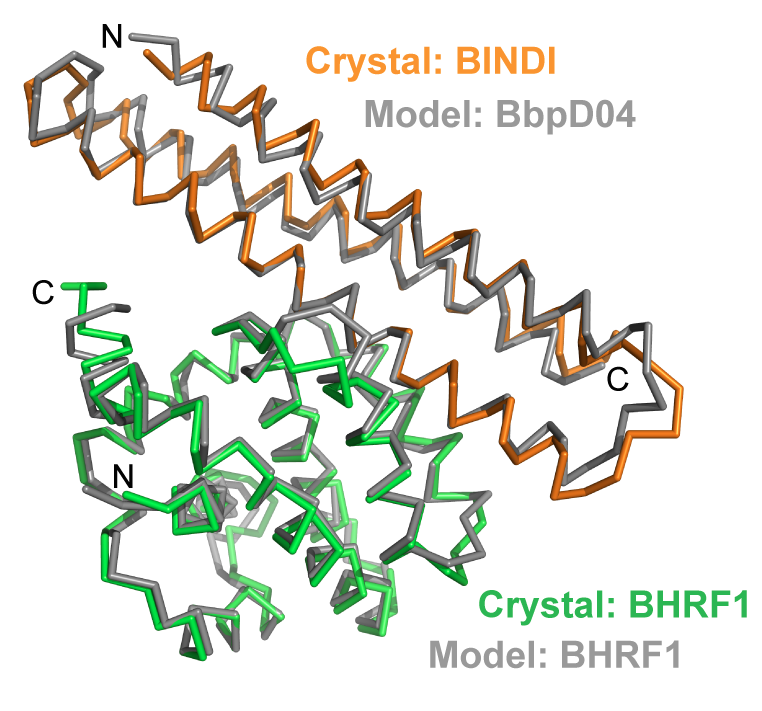
Since apopotosis of infected cells can limit virus production and spread, some viruses have co-opted prosurvival genes from the host. This includes the Epstein-Barr virus gene BHRF1, a homologue of human Bcl-2 proteins that block apoptosis and are associated with cancer. Here we describe an approach where computational design and experimental optimization were used to generate a novel protein called BINDI that binds BHRF1 with picomolar affinity. BINDI recognizes the hydrophobic cleft of BHRF1 in a similar manner to other Bcl-2 protein interactions, but makes many additional contacts to acheive exceptional affinity and specificity. BINDI induces apoptosis in EBV-infected cancer lines, and when delivered with an antibody-targeted intracellular delivery carrier, BINDI suppressed tumor growth and extended survival in a xenograft disease model of EBV-positive human lymphoma. High specificity designed proteins that selectively kill target cells may provide an advantage over the toxic compounds used in current generation antibody-drug conjugates.
Procko, E. et al. Cell 157, 1644-1656. (2014)