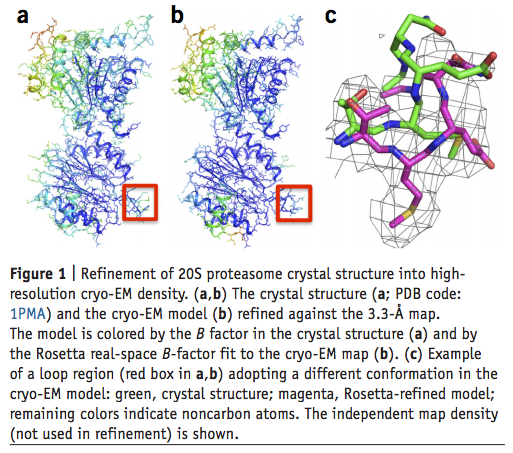
We describe a general approach for refining protein structure models on the basis of cryo-electron microscopy maps with near-atomic resolution. The method integrates Monte Carlo sampling with local density-guided optimization, Rosetta all-atom refinement and real-space B-factor fitting. In tests on experimental maps of three different systems with 4.5-Å resolution or better, the method consistently produced models with atomic-level accuracy largely independently of starting-model quality, and it outperformed the molecular dynamics-based MDFF method. Cross-validated model quality statistics correlated with model accuracy over the three test systems (20S proteasome, PrgH/PrgK periplasmic domains, and peptide fiber).
DiMaio, F et al. Nature Methods 12(4), 361-365 (2015)