King, N.P., Sheffler, W., et al. Science. 336(6085), 1171-1174. (2012)
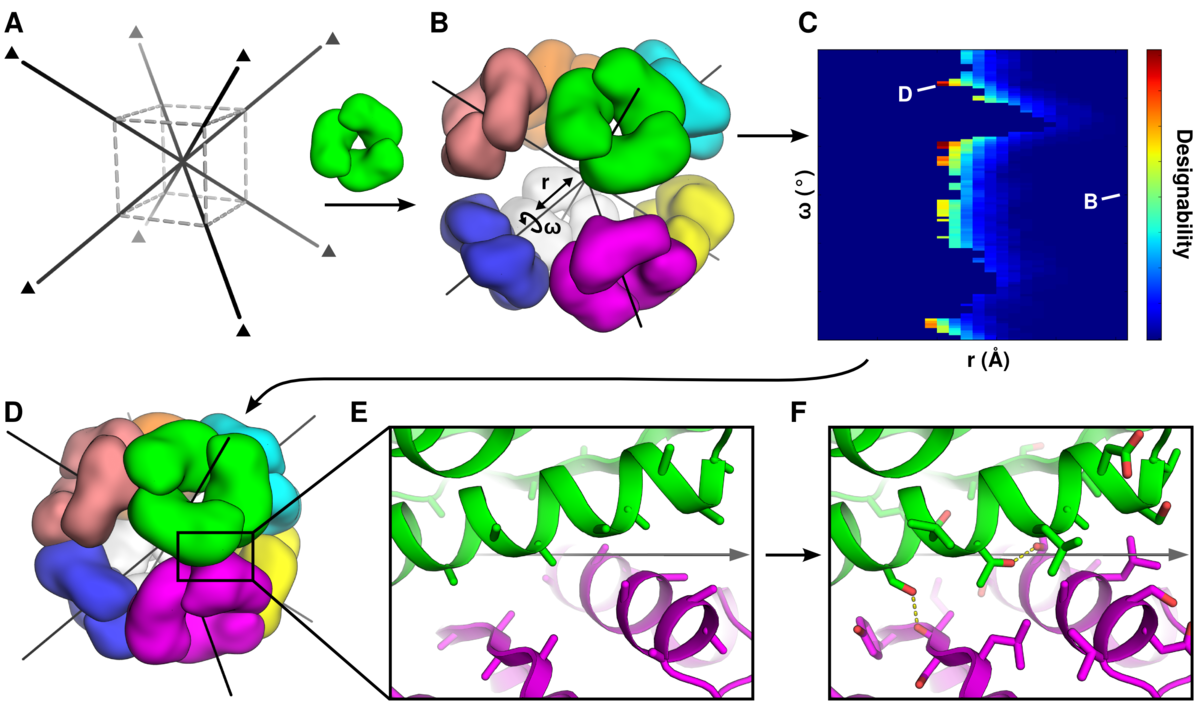
We describe a general computational method for designing proteins that self-assemble to a desired symmetric architecture. Protein building blocks are docked together symmetrically to identify complementary packing arrangements, and low-energy protein-protein interfaces are then designed between the building blocks in order to drive self-assembly. Here we use trimeric protein building blocks to design a 24-subunit, 13 nm diameter complex with octahedral symmetry and two related variants of a 12-subunit, 11 nm diameter complex with tetrahedral symmetry. The designed proteins assembled to the desired oligomeric states in solution, and crystal structures of the complexes revealed that the resulting materials closely match the design models. The method can be used to design a wide variety of self-assembling protein nanomaterials.